Introduction
The complex and challenging pancreaticoduodenectomy,
sometimes called the “Whipple operation” is typically performed on elderly patients with pancreatic cancer and periampullary diseases. Younger patients are rarely given pancreaticoduodenectomy procedures, and the impact of age on surgical
and survival outcomes is still unclear [1].
Patients in their 30s or 40s are rarely found to have pancreatic duct adenocarcinoma, which is often detected in patients
aged 65-75 years of age [2,3]. The influence of youth on surgical
and survival outcomes following pancreaticoduodenectomy has
not been thoroughly investigated, given its uncommon occurrence in younger patients. There is little literature in this field
[1-5].
Traditionally, an open technique is used to perform pancreaticoduodenectomy using a high abdominal incision, right saber
slash, or a lengthy upper midline incision. This leads to severe
pain and sometimes even negative outcomes [6]. Minimally Invasive Surgery (MIS) has become the norm in several specialties, including pancreatic resection, because of reduced pain,
improved cosmesis, and smaller incisions. According to certain
findings, older people can have Laparoscopic Pancreaticoduodenectomy (LPD) with good results [7-9]. However, pancreaticoduodenectomy entails precise identification of the vital vascular anatomy, considerable dissection and removal of visceral
organs, and technically challenging repair. As a result, minimally
invasive pancreaticoduodenectomy is not as widely used [10].
Recently, robotic surgery has emerged to overcome the limitations of laparoscopic surgery, following the release of the Intelligent Surgical®, Sunnyvale, California, USA, da Vinci Robotic
Surgical Machine. This technology can offer tremor-free movements for both cams and tools, high-quality 3-Dimensional view
of the surgical field, and end wrist devices to enhance the spectrum of flexibility emulating open procedures.
These developments help lessen surgeon fatigue, enhance
ergonomics, and increase dexterity, but less used as its high
patients cost and less surgeon experiences [10,11]. Although
the robotic method of pancreaticoduodenectomy has been
adopted slowly, a number of studies have demonstrated that
RPD is a safe and viable technique compared with laparotomy
[6,10,12,13].
The majority of the information on surgical outcomes and
treatment choices that is currently accessible comes from studies conducted on older populations, as there is little research on
pancreaticoduodenectomy in younger individuals. Therefore, it
is unclear how younger and older groups differ in terms of tumor biology and surgical results. To date, there have we been
no studies on RPD in younger groups. To better understand the
clinicopathological characteristics, surgical outcomes, and survival outcomes of young patients (less than 50 years old) undergoing RPD, our study compared them with an older patient
cohort (>50 years old) undergoing RPD at our institute.
Patients and methods
Patient choice
The study comprised patients who underwent RPD at five
surgical institutes between Jan 2012 - Oct 2023 and data collected at our institute, 37 cases later, the learning curve for
the RPD was surmounted. The first RPD was completed on Jan.
2012.
Division of the patients into two categories based on the age,
RPD: young (less than 50 years) and old (>50 years). Any patient
had history of operation with marked adhesion >2 cm specially
upper part of the abdomen were excluded from our work.
Data gathering
All data related to patients character and tumor features
were collected, patients classified physically by the American
Society of Anesthesiologists (ASA). All Preoperative , intraoperative and post operative morbidity were detected and gathered.
The likhookvarible associated with surgery, such as fatality and
different postoperative difficulties, were also evaluated. Periampullary adenocarcinoma death incidence have also been reported.
Aim of study outcomes
Primary aim of the study to contrast the safety and risks of
our cases categories. The secondary study goal is survival comparison between both.
Method procedures
A brief internal stent was inserted for a small pancreatic duct
measuring less than 3 mm, although pancreatic duct stents are
not commonly employed. The same jejunal limb was then used
for hepaticojejunostomy without stenting, either with continuous (for dilated) or interrupted (for non dilated ducts) sutures.
By carefully lowering the stomach, an extracorporeal technique
was used to execute hand-sewn gastrojejunostomy. The gastrojejunostomy was placed in framesocolic, antecolic, and antiperistaltic positions close to the umbilical region. When feasible, a restricted antrectomy was received following right gastric
artery bifercation in patients with an ischemic pylorus instead
of attempting pylorus-preserving pancreaticoduodenectomy.
Oral liquid after 24 h and soft diet after 3 days, no need for NGT
feeding.
The Clavien-Dindo classification was used to categorize surgical complications [14]. According to the 2016 International
Study Group for Pancreatic Fistula revised grading system [15],
clinically meaningful grade B or C pancreatic leakage constitutes
the definition of Postoperative Pancreatic Fistula (POPF). The
International Study Group of Pancreatic Surgery (ISGPS) established classification criteria for Delayed Gastric Emptying (DGE),
Post-Pancreatectomy Hemorrhage (PPH), and chyle leak [16-18].
Based on the state of the resection margin, compelete radical resection was we had three degree: If there was no microscopic evidence of cancer at a resection margin of less than 1
mm, the resection was classified as R0; if there was microscopic
evidence of cancer at a resection margin of less than 1 mm,
it was classified as R1; and if there was strong positive margin, it was classified as R2. Mortality that occurs through three
months following surgery, involving hospitalization period after
surgery, is referred to as surgical mortality.
Data statistics
The statistical product and service solutions version 26 program was used to perform the statistical analysis. Continuous
variables were compared using a two-tailed student’s t-test
and expressed as mean ± standard deviation. The Wilcoxon
rank-sum test was used for continuous variables that were not
normally distributed. Categorical variables are represented as
numbers (percentages), and Pearson’s χ2 test or Fisher’s exact test contingency tables were used to compare them. The overall
survival between the young and old groups was compared using Kaplan-Meier survival curves, and significance was assessed
using the log-rank test. Cox proportional hazards regression and
binary logistic regression were used for the multivariate analysis. Statistical significance was set at P<0.05.
Table 1: Shows the demographics of the patients who underwent robotic pancreaticoduodenectomy with periampullary lesions.
|
Total |
Age <50 y/o |
Age ≥50 y/o |
P value |
| Patients, n(%) |
555 |
53(9.5%) |
502(90.5%) |
|
| Age, year old |
|
|
|
<0.001 |
| Median (range) |
67(13-97) |
42(13-49) |
68(50-97) |
|
| Mean±SDa |
66±12 |
40±9 |
69±9 |
|
| Sex |
|
|
|
0.512 |
| female |
259(46.7%) |
27(50.9%) |
232(46.2%) |
|
| male |
296(53.3%) |
26(49.1%) |
270(53.8%) |
|
| BMIb, kg/m2 |
|
|
|
0.628 |
| Median (range) |
23.5(15.4–36.2) |
23.1(16.7-34.1) |
23.5(15.4-36.2) |
|
| Mean±SD |
23.7±3.5 |
23.9±4.1 |
23.7±3.4 |
|
| ASAc physical status classification |
|
|
<0.001 |
| <3 |
359(64.7%) |
48(90.6%) |
311(62.0%) |
|
| ≥3 |
196(35.3%) |
5(9.4%) |
191(38.0%) |
|
| Periampullary lesions |
|
|
|
<0.001 |
| Pancreatic head adenocarcinoma |
193(34.8%) |
7(13.2%) |
186(37.1%) |
|
| Ampullary adenocarcinoma |
139(25.0%) |
6(11.3%) |
133(25.5%) |
|
| Distal CBDdadenocarcinoma |
43(7.7%) |
0(0.0%) |
43(8.6%) |
|
| Duodenal adenocarcinoma |
26(4.7%) |
4(7.5%) |
22(4.4%) |
|
| IPMNe |
43(7.7%) |
4(7.5%) |
39(7.8%) |
|
| Neuroendocrine tumor |
26(4.7%) |
8(15.1%) |
18(3.6%) |
|
| Solid and pseudopapillary tumor |
10(1.8%) |
5(9.4%) |
5(1.0%) |
|
| Chronic pancreatitis |
16(2.9%) |
5(9.4%) |
11(2.2%) |
|
| Other malignant tumor |
33(5.9%) |
7(13.2%) |
26(5.2%) |
|
| Other benign tumor |
26 (4.7%) |
7(13.2%) |
19(3.8%) |
|
| Periampullary adenocarcinomas |
|
|
<0.001 |
| Yes |
401(72.3%) |
17(32.1%) |
384(76.5%) |
|
| No |
154(27.7%) |
36(57.9%) |
118(23.5%) |
|
| Periampullary adenocarcinomas |
|
|
0.626 |
| Pancreatic head adenocarcinomas |
193(48.1%) |
7(41.2%) |
186(48.4%) |
|
| Other periampullary adenocarcinoma |
208(51.9%) |
10(58.8%) |
198(51.6%) |
|
| Pancreatic parenchyma |
|
|
|
0.033 |
| soft |
355(64.0%) |
41(77.4%) |
314(62.5%) |
|
| hard |
200(36.0%) |
12(22.6%) |
188(37.5%) |
|
| Pancreatic duct |
|
|
|
<0.001 |
| non-dilated ≤3 mm |
270(49.3%) |
41(77.4%) |
229(46.3%) |
|
| dilated >3 mm |
278(50.7%) |
12(22.6%) |
266(53.7%) |
|
| Tumor size, cm |
|
|
|
0.263 |
| Median (range) |
3.0(0.5-11.0) |
3.0(1.0-8.5) |
3.0(0.5-11.0) |
|
| Mean±SD |
3.1±1.4 |
3.3±1.7 |
3.1±1.4 |
|
aSD: standard deviation; bBMI: body mass index; cASA: American Society of Anesthesiologists; dCBD: common bile duct; eIPMN: intraductal papillary
mucinous neoplasm
Results
This study included 555 patients; 53(9.5%) belonged to
the younger group (age <50 years), whereas 502(90.5%) belonged to the older group (age ≥50 years) (Table 1). Regarding
the demographics of the two groups, there were no notable
differences in terms of sex, BMI, or tumor size. Nonetheless,
a greater percentage of patients in the younger cohort were
classified as having an ASA physical status ≥3 (9.4% vs. 38.0%,
p<0.001). Periampullary adenocarcinomas were less common
in the younger group than in the older group (32.1% vs 76.5%,
p<0.001). However, solid and pseudopapillary tumors (9.4% vs.
1.0%) and neuroendocrine tumors (15.1% vs. 3.6%) were more
common in the younger patients. Two types of periampullary
adenocarcinoma were found in the same number of young and
old people (p=0.626): Periampullary adenocarcinoma in the
pancreatic head (41.2% vs. 48.4%) and other types (58.8% vs.
51.6%). Some pancreatic ducts were not dilated (≤3 mm) more
often in the younger group (77.4% vs. 46.3%, p < 0.001), and the
pancreatic parenchyma was softer (77.4% vs. 62.5%, p=0.033).
In terms of surgical outcomes (Table 2), there were no statistically significant differences between the young and old groups
in terms of operation time (median, 7.8 vs. 8.3 h; p=0.508), intraoperative blood loss (median, 100 vs. 160 mL; p=0.681), surgical radicality (R0 resection, 92.5% vs. 85.1%; p=0.217), lymph
node yield (median, 17 vs. 18; p=0.681), lymph node involvement (50.0% vs. 56.1%, p=0.798), stage 1 + 2 (58.8% vs. 70.6%, p = 0.292), conversion rate (5.7 vs. 8.4%, p = 0.492), and vascular
resection rate (3.8% vs. 3.8%, p = 0.997). In the younger group,
the majority of the surgical outcomes were positive. Compared
to the senior group (median of 20 days), the LOS of the young
group was shorter (median of 16 days; p = 0.033). Age by itself
was not an independent predictor of longer length of stay (LOS)
following RPD, although pancreatic head adenocarcinoma (+),
morbidity (+), POPF (+), and chyle leakage (+) were observed on
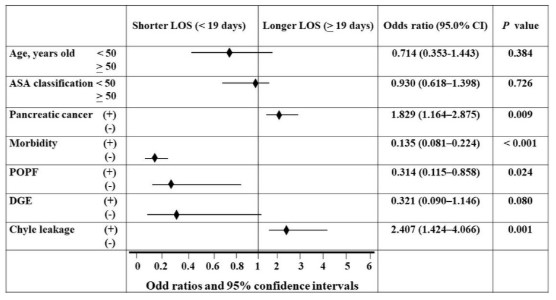
multivariate analysis using binary logistic regression (Figure 1).
The Length of Stay (LOS) following robotic pancreaticoduodenectomy was predicted by the independent components, as
shown in Figure 1’s forest plot of multivariate analysis using binary logistic regression. US Society of Anesthesiologists (ASA);
Reliability interval (CI); Postoperative Pancreatic Fistula (POPF);
Delayed Gastric Emptying (DGE).
Table 2: Surgical results following pancreaticoduodenectomy using robotics.
|
Total |
Age <50 y/o |
Age ≥50 y/o |
P value |
| Patients, n |
555 |
53(9.5%) |
502(90.5%) |
|
| Operation time, hour |
|
|
|
0.508 |
| Median (range) |
8.0(3.3-16.3) |
7.8(4.0-13.5) |
8.3(3.3-16.3) |
|
| Mean±SDa |
8.4±2.3 |
7.9±2.3 |
8.4±2.3 |
|
| Blood loss, c.c. |
|
|
|
0.681 |
| Median (range) |
160(0-6000) |
100(0-4600) |
160(0-6000) |
|
| Mean±SD |
239±396 |
261±666 |
237±357 |
|
| Surgical radicality |
|
|
|
0.217 |
| R0 |
476(85.8%) |
49(92.5%) |
427(85.1%) |
|
| R1 |
57(10.3%) |
4(7.5%) |
53(10.6%) |
|
| R2 |
22(4.0%) |
0 |
22(4.4%) |
|
| Lymph node yield |
|
|
|
0.351 |
| Median (range) |
18(12-49) |
17(12-37) |
18(12-49) |
|
| Mean±SD |
19±6 |
18±6 |
19±5 |
|
| Lymph node involvement |
218(55.9%) |
8(50.0%) |
210(56.1%) |
0.798 |
| Stage |
|
|
|
0.292 |
| 1+2 |
281(70.1% |
10(58.8%) |
271(70.6%) |
|
| 3+4 |
120(29.9%) |
7(41.2%) |
113(29.4%) |
|
| Conversion to open, n (%) |
45(8.1%) |
3(5.7%) |
42(8.4%) |
0.492 |
| Vascular resection, n (%) |
21(3.8%) |
2(3.8%) |
19(3.8%) |
0.997 |
| LOSb, day |
|
|
|
0.033 |
| Median (range) |
19(6-118) |
16(6-46) |
20(6-118) |
|
| Mean±SD |
23±14 |
19±9 |
23±14 |
|
aSD: standard deviation; bLOS: length of stay
Table 3: Risks associated with surgery following robotic pancreaticoduodenectomy
|
Total |
Age <50 y/o |
Age ≥50 y/o |
P value |
| Patients, n |
555 |
53(9.5%) |
502(90.5%) |
|
| Surgical mortality |
8(1.5%) |
0 |
8(1.6%) |
0.352 |
| Surgical morbidity |
312(56.2%) |
28(52.8%) |
284(56.6%) |
0.601 |
| Surgical complication |
|
|
|
0.888 |
| Clavien–Dindo 0 |
236(42.5%) |
23(43.4%) |
213(42.4%) |
|
| Clavien–Dindo I |
191(34.4%) |
18(34.0%) |
173(34.5%) |
|
| Clavien–Dindo II |
52(9.4%) |
5(9.4%) |
47(9.4%) |
|
| Clavien–Dindo III |
62(11.2%) |
7(13.2%) |
55(11.0%) |
|
| Clavien–Dindo IV |
5(0.9%) |
0 |
5(1.0%) |
|
| Clavien–Dindo V (death) |
9(1.6%) |
0 |
9(1.8%) |
|
| Severity of complication, n=319 |
|
|
|
0.947 |
| Minor (Clavien–Dindo I-II) |
243(76.2%) |
23(76.7%) |
220(76.1%) |
|
| Major (Clavien–Dindo≥III) |
76(23.8%) |
7(23.3%) |
69(23.9%) |
|
| POPFa (ISGPFb grade B and C) |
| Overall |
44(7.9%) |
4(7.5%) |
40(8.0%) |
0.914 |
| Parenchyma of pancreas |
| soft |
37(10.4%) |
4(9.8%) |
33(10.5%) |
0.882 |
| hard |
7(3.5%) |
0 |
7(3.7%) |
0.496 |
| Diameter of pancreatic duct |
| non-dilated ≤3 mm |
30(11.1%) |
4(9.8%) |
26(11.4%) |
0.746 |
| dilated >3 mm |
14(5.0%) |
0 |
14(5.3%) |
0.415 |
| DGEc (ISGPSd grade B and C) |
24(4.3%) |
1(1.9%) |
23(4.6%) |
0.359 |
| PPHe (ISGPSd grade B and C) |
32(5.8%) |
4(7.5%) |
28(5.6%) |
0.559 |
| Chyle leakage |
140(25.2%) |
14(26.4%) |
126(25.1%) |
0.834 |
| Bile leakage |
10(1.8%) |
1(1.9%) |
9(1.8%) |
0.961 |
| Wound infection |
28(5.0%) |
1(1.9%) |
27(5.4%) |
0.269 |
aPOPF: Postoperative Pancreatic Fistula, bISGPF: International Study Group of Pancreatic Fistula; cDGE: Delayed Gastric Emptying; dISGPS: International Study Group of Pancreatic Surgery; ePPH: Postpancreatectomy Hemorrhage.
With no surgical mortality in the young group and 1.6% in
the old group (p=0.352), the cohort’s overall surgical mortality
rate was 1.5%. All patients had a DGE rate of 4.3%:1.9% in the
young group and 4.6% in the old group (P=0.359). With 7.5% in
the young group and 8.0% in the old group, the overall POPF
rate was 7.9% (P=0.914). Additionally, there were no appreciable differences between the younger and older groups in terms
of surgical morbidity, Clavien-Dindo surgical complications, severity of problems, PPH, chyle leakage, bile leakage, or wound
infection (Table 3).
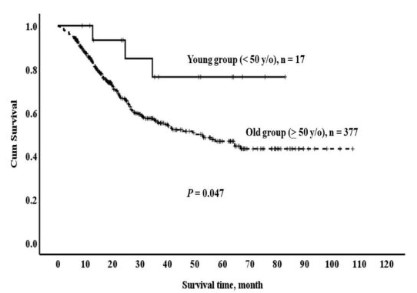
Regarding survival results, 48.1% of the total cohort with
periampullary adenocarcinomas survived for five years (Table
4). In terms of overall periampullary adenocarcinoma, the
5-year survival rate of the younger group was considerably
higher than that of the older group (76.4% vs. 46.7%, p=0.047)
(Figure 2). The 5-year survival rates for ampullary adenocarcinoma and pancreatic head adenocarcinoma were 100% vs. 61.4%
(p=0.159) and 62.5% vs. 31.4% (p=0.171), respectively. However, there was no clear difference between the two groups in
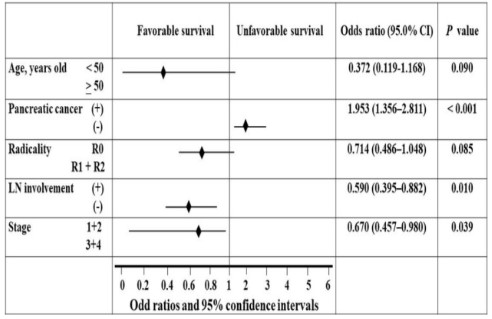
terms of survival rates. The Cox proportional hazards regression
model (Figure 3) showed that age was not a reliable predictor
of poor survival after robotic pancreaticoduodenectomy. However, pancreatic head cancer (+), Lymph Node (LN) involvement
(+), and late stage 3+4 (+) were observed.
To estimate how long someone will live after a robotic pancreaticoduodenectomy, we used the Cox proportional hazards
regression model and the forest plot in Figure 3 to identify independent prognostic factors.
Table 4: Survival rates following robotic pancreaticoduodenectomy for periampullary adenocarcinomas.
| Periampullary adenocarcinoma |
Median, (mon.) |
Range, (mon.) |
Mean ± SDa, (mon.) |
1-year survival |
3-year survival |
5-year survival |
P value |
| Overall periampullary |
|
|
|
|
|
|
|
| Total, n=394 |
20.4 |
0.2-107.6 |
28.7+23.3 |
85.4% |
57.1% |
48.1% |
0.047 |
| Age <50 y/o, n=17 |
35.3 |
8.9-82.9 |
40.1±24.2 |
100% |
76.4% |
76.4% |
|
| Age ≥50 y/o, n=377 |
20.2 |
0.2-107.6 |
28.2±23.2 |
84.7% |
56.2% |
46.7% |
|
| Pancreatic head |
|
|
|
|
|
|
|
| Total, n=191 |
16.6 |
0.8-98.1 |
23.0±19.8 |
77.8% |
40.4% |
32.9% |
0.171 |
| Age <50 y/o, n=7 |
24.6 |
8.9-67.3 |
34.4±22.4 |
100% |
62.5% |
62.5% |
|
| Age ≥50 y/o, n=184 |
16.5 |
0.8-98.1 |
22.6±19.6 |
76.9% |
39.4% |
31.4% |
|
| Ampullary |
|
|
|
|
|
|
|
| Total, n=136 |
28.1 |
0.2-107.6 |
35.7±26.3 |
91.3% |
73.9% |
63.1% |
0.159 |
| Age <50 y/o, n=6 |
43.4 |
11.7-75.6 |
41.9±29.0 |
100% |
100% |
100% |
|
| Age ≥50 y/o, n=130 |
28.1 |
0.2-107.6 |
35.7±26.3 |
90.9% |
72.8% |
61.4% |
|
aSD: standard deviation.
Discussion
Given that less than 30% of tumors are projected to arise in
young people, pancreatic cancer and other periampullary malignancies are uncommon among younger patients compared
to older patients [19]. This is particularly true for pancreatic
cancer and other periampullary malignancies. Malignancies in
young people may differ from those in the elderly in terms of
their molecular characteristics and tumor biology. Although
there is debate about whether young patients have a worse
prognosis than older patients, our present understanding of
cancer in this population is inadequate [2]. Furthermore, the
implementation of MIS occurs more frequently. Nevertheless,
little research has been conducted on how early age affects surgery and survival after RPD.
Periampullary adenocarcinomas were less common in the
younger group (32.1% vs. 76.5%) than in the older group. In
contrast, the younger group had higher rates of solid and pseudopapillary tumors (9.4% vs. 1.0%) and neuroendocrine tumors
(15.1% vs. 3.6%). In a study of pancreaticoduodenectomy in a
young population (≤30 years old), Mansfield et al. [20] discovered that chronic pancreatitis (6, 27.3%) was the most common
postoperative histologic diagnosis, followed by solid pseudopapillary tumors (22.7%) and adenocarcinomas (18.2%). A case
series of young adults (less than 35 years) who underwent pancreaticoduodenectomy was described by El Nakeeb et al. [1].
The results showed that adenocarcinoma (41.4%) was the most
common pathological diagnosis in this cohort, followed by solid
pseudopapillary tumors (29.3%). Although the most common diagnosis reported in the literature is inconsistent, solid pseudopapillary tumors have become a common histological diagnosis in young individuals.
Younger people may be more susceptible to pancreatic leakage because they often have a smaller pancreatic duct, a less
fibrotic pancreas, and a more normal pancreatic parenchyma.
As predicted, the prevalence of non-dilated (<3 mm) pancreatic
ducts and soft pancreatic parenchyma was higher in the younger group (77.4% vs. 62.5% and 77.4% vs. 46.3%, respectively).
Despite these variations, the younger group did not have an
increase in POPF or surgical complications compared with the
older group. Furthermore, there was no surgical mortality in the
younger group, supporting the findings of other studies [1,5,20]
that RPD are safe for young patients. Although the youth group
in this study had a shorter LOS (median: 16 vs. 20 days), age by
itself was not an independent predictor of LOS following multivariate analysis. Most likely, reduced morbidity, lower POPF,
and fewer cases of pancreatic head adenocarcinoma contributed to the shorter LOS in younger patients.
There is ongoing discussion regarding the relative aggressiveness of younger versus older patients with pancreatic duct
adenocarcinomas [2-5]. Meng et al. [5] found no significant correlation between age and long-term survival in patients with
pancreatic and periampullary adenocarcinomas after LPD. Additionally, Yeh et al. [21] showed that, following pancreaticoduodenectomy, actuarial survival was comparable between older
and younger patients. According to several experts, cancer in
older adults might be less aggressive biologically [22,23]. Consequently, it is believed that younger cancer patients have a
poorer prognosis than older ones [24-27]. After radical resection
of pancreatic ductal adenocarcinoma, Tang et al. [2] compared
adolescents and young adults using propensity score matching and concluded that the disease may be more aggressive in
these age groups. Additionally, Mansfield et al. [20] found that
the median survival for juvenile adenocarcinoma patients was
10.2 months, compared to 57.8 months for adult patients. However, El Nakeeb et al. [1] demonstrated that the median survival of young adult patients with pancreatic adenocarcinoma
was much better than that of older patients, in contrast to the
findings of Tang and Mansfield [2,20]. In this investigation, the
younger group outperformed the older group by five years for
total periampullary adenocarcinoma (76.4% vs. 46.7%). In both the ampullary and pancreatic head adenocarcinoma groups,
there was a trend toward improved survival outcomes in the
younger group, although the difference was not statistically significant. Age was not an independent predictive factor for periampullary adenocarcinoma after multivariate analysis. This may
be because our study included fewer cases of pancreatic head
cancer and lymph node involvement. Nevertheless, the small
sample size of the young group made it difficult to reach firm
conclusions. Larger sample sizes and additional research are required to validate these results and to explore the underlying
mechanisms.
This study had certain shortcomings. Initially, all adult patients were included in the older cohort regardless of their
comorbidities or overall health. Second, the small sample size
of the young group makes statistical errors more likely and restricts our ability to properly grasp biological aggression.
Conclusions
Individuals under 50 years of age can safely undergo RPD,
and their surgical results will be similar to those of older individuals. Additionally, although the results were not independent,
younger patients with periampullary adenocarcinoma showed
considerably better survival outcomes than older patients.
These results lend credence to the viability and possible advantages of RPD in the pediatric population. Further investigation
with a larger sample size is required to verify these results and
to investigate the underlying mechanisms.
Declarations
Acknowledgment: Not applicable.
Declaration and ethical clearance: Ethical clearance was
obtained from Zagagic University, Faculty of Medicine, Institutional Health Research Ethics (IHRERC), and written informed
consent was obtained from the Review IHRERC under No. (ethical protocol number ZU-IRB#99902792023). Written informed
consent was obtained from the Zagazig University Hospital database in accordance with the Declaration of Helsinki.
Consent for publication: Not applicable.
Availability of data and materials: A database is available to
the corresponding author. This database is available upon review and request. All authors shared the database.
Competing interests: The authors declare that they have no
competing interests or financial disclosures.
Funding: No specific funds were received for this study
References
- El Nakeeb A, El Sorogy M, Salem A, Said R, El Dosoky M, et al.
Surgical outcomes of pancreaticoduodenectomy in young patients: A case series. Int J Surg. 2017; 44: 287-294.
- Tang N, Dou X, You X, Liu G, Ou Z, et al. Comparisons of Outcomes Between Adolescent and Young Adult with Older Patients
After Radical Resection of Pancreatic Ductal Adenocarcinoma by
Propensity Score Matching: A Single-Center Study. Cancer Manag Res. 2021; 13: 9063-9072.
- Barbas AS, Turley RS, Ceppa EP, Reddy SK, Blazer DG, et al. Comparison of outcomes and the use of multimodality therapy in
young and elderly people undergoing surgical resection of pancreatic cancer. J Am Geriatr Soc. 2012; 60: 344-350.
- Liu Q, Zhao Z, Zhang X, Zhao G, Tan X, et al. Robotic pancreaticoduodenectomy in elderly and younger patients: A retrospective
cohort study. Int J Surg. 2020; 81: 61-65.
- Meng L, Xia Q, Cai Y, Wang X, Li Y, et al. Impact of Patient Age
on Morbidity and Survival Following Laparoscopic Pancreaticoduodenectomy. Surg Laparosc Endosc Percutan Tech. 2019; 29:
378-382.
- Shyr BU, Shyr BS, Chen SC, Shyr YM, Wang SE. Mesopancreas
level 3 dissection in robotic pancreaticoduodenectomy. Surgery.
2021; 169: 362-368.
- Kim JS, Choi M, Kim SH, Choi SH, Kang CM. Safety and feasibility of laparoscopic pancreaticoduodenectomy in octogenarians.
Asian J Surg. 2022; 45: 837-843.
- Chapman BC, Gajdos C, Hosokawa P, Henderson W, Paniccia A,
et al. Comparison of laparoscopic to open pancreaticoduodenectomy in elderly patients with pancreatic adenocarcinoma.
Surg Endosc. 2018; 32: 2239-2248.
- Jones LR, Zwart MJW, Molenaar IQ, Koerkamp BG, Hogg ME, et
al. Robotic Pancreatoduodenectomy: Patient Selection, Volume
Criteria, and Training Programs. Scand J Surg. 2020; 109: 29-33.
- Mantzavinou A, Uppara M, Chan J, Patel B. Robotic versus open
pancreaticoduodenectomy, comparing therapeutic indexes; a
systematic review. Int J Surg. 2022; 101: 106633.
- van Oosten AF, Ding D, Habib JR, Irfan A, Schmocker RK, et al.
Perioperative Outcomes of Robotic Pancreaticoduodenectomy:
A Propensity-Matched Analysis to Open and Laparoscopic Pancreaticoduodenectomy. J Gastrointest Surg. 2021; 25: 1795-
1804.
- Shyr BU, Chen SC, Shyr YM, Wang SE. Surgical, survival, and oncological outcomes after vascular resection in robotic and open
pancreaticoduodenectomy. Surg Endosc. 2020; 34: 377-383.
- Wang SE, Shyr BU, Chen SC, Shyr YM. Comparison between
robotic and open pancreaticoduodenectomy with modified
Blumgart pancreaticojejunostomy: A propensity score-matched
study. Surgery. 2018; 164: 1162-1167.
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, et al.
The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 2009; 250: 187-196.
- Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, et al.
The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years
After. Surgery. 2017; 161: 584-591.
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al.
Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic
Surgery (ISGPS). Surgery. 2007; 142: 761-768.
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, et al. Post-pancreatectomy hemorrhage (PPH): An International Study
Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;
142: 20-25.
- Besselink MG, van Rijssen LB, Bassi C, Dervenis C, Montorsi M,
et al. Definition and classification of chyle leak after pancreatic
operation: A consensus statement by the International Study
Group on Pancreatic Surgery. Surgery. 2017; 161: 365-372.
- Langan RC, Huang CC, Mao WR, Harris K, Chapman W, et al. Pancreaticoduodenectomy hospital resource utilization in octoge-
narians. Am J Surg. 2016; 211: 70-75.
- Mansfield SA, Mahida JB, Dillhoff M, Porter K, Conwell D, et al.
Pancreaticoduodenectomy outcomes in the pediatric, adolescent, and young adult population. J Surg Res. 2016; 204: 232-
236.
- Yeh CC, Jeng YM, Ho CM, Hu RH, Chang HP, et al. Survival after
pancreaticoduodenectomy for ampullary cancer is not affected
by age. World J Surg. 2010; 34: 2945-2952.
- Fisher CJ, Egan MK, Smith P, Wicks K, Millis RR, et al. Histopathology of breast cancer in relation to age. Br J Cancer. 1997; 75:
593-596.
- Monson K, Litvak DA, Bold RJ. Surgery in the aged population:
surgical oncology. Arch Surg. 2003; 138: 1061-1067.
- Cho SJ, Yoon JH, Hwang SS, Lee HS. Do young hepatocellular carcinoma patients with relatively good liver function have poorer
outcomes than elderly patients? J Gastroenterol Hepatol. 2007;
22: 1226-1231.
- Emile SH, Elfeki H, Shalaby M, Elbalka S, Metwally IH, et al. Patients with early-onset rectal cancer aged 40 year or less have
similar oncologic outcomes to older patients despite presenting
in more advanced stage; A retrospective cohort study. Int J Surg.
2020; 83: 161-168.
- Llanos O, Butte JM, Crovari F, Duarte I, Guzmán S. Survival of
young patients after gastrectomy for gastric cancer. World J
Surg. 2006; 30: 17-20.
- Nakamura R, Saikawa Y, Takahashi T, Takeuchi H, Asanuma H, et
al. Retrospective analysis of prognostic outcome of gastric cancer in young patients. Int J Clin Oncol. 2011; 16: 328-334.