Introduction
Carcinoid (neuroendocrine neoplasm) is a rare type of neoplasm. Most of these tumors derive primarily from the gastro-intestinal tract (55%) and the bronchopulmonary segments
(30%) [1] but are capable of arising throughout the body. Occurrence of the intracranial carcinoid is very rare, only 4 cases
have been reported in the literature [2-5]. Of them, first case
was recognized as a mass with a prominent dural-based tail that
compressed the right frontal lobe. Second case was confirmed
as a mass located at the foramen jugulare extending into the
cerebello-medullary angle cistern. Third case was revealed as a
tumor in the cerebello-pontine angle. For the fourth case, the
tumor was present at a cavernous sinus extending into the infratemporal fossa. Thus, location of these carcinoids was variable and they were not collision types. However, considering
present case in addition of these reports, skull base area will
be one of preferable sites for the occurrence of carcinoid tumors. In this case, carcinoid coexisted with Rathke’s cleft cysts
(RCCs) known as pars intermedia cysts, represent benign lesions
formed from remnant of the embryologic Rathke’s pouch. The
incidence of RCCs varies from 2 to 26% as seen in autopsy series
[6,7]. Coexistence of RCCs and pituitary adenomas is already
notable [8-10].
Concerning the pathogenesis of collision of RCCs and pituitary adenoma, Kepes [11] exhibited a transitional cell tumor
of the pituitary gland developing from a RCCs, considering that the tumor was derived from “transitional” cells between the
lining cells of RCCs and the glandular cells of the anterior pituitary. However, the theory was rejected by Ikeda et al [12]
who proved that the tumor shown by Kepes [11] corresponds
to an early development stage of the pituitary anterior lobe and
that a cyst within a pituitary adenoma differs from cyst found in
the embryonic stage of the pituitary gland. Thus, pathogenesis
of the collision of pituitary adenoma and RCCs is still controversial. Presently, we report a collision case of carcinoid tumor
and RCCs. So far as we concerned, such collision case has never
been described. Certainly, the relationship between the two lesions is now unknown. Nevertheless, our report will be important for understanding of sellar lesions including neoplasms and
non-neoplastic cystic lesions.
Case presentation
A 57-year-old man received a medical checkup at medical
checkup center of our hospital. After the CT examination as a
course of the checkup, the patient was notified a possibility of
neoplasm of pituitary gland, although hormonal inbalance was
not present. Neurological examination including visual activity
and visual fields showed no abnormality. Since he did not have
any symptoms suggesting intracranial neoplasm, a follow up of
observation by the group of neurosurgery of our hospital was
decided. About 5 years later, he started to develop consistent
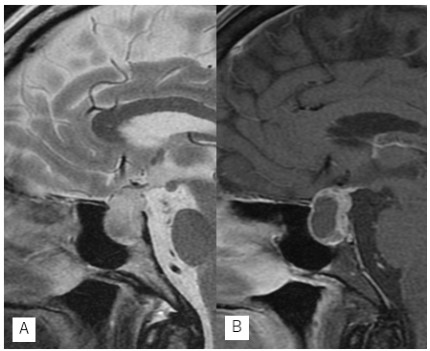
headache. Magnetic resonance imaging revealed a cystic sellar
mas which was slightly hypertense with sparse hypointence in
the lesion on T2-weighted image (Figure 1A) and was enhancing
after the administration of gadolinium (Figure 1B). These evidences suggest to indicate that cystic mass gradually grew up
from 20 mm to 25 mm in 5 years.
The patient underwent an endoscopic endonasal transsphenoidal surgery (eTSS). During the procedure of eTSS, jerry-like
fluid was discharged implying the presence of RCCs. Histologically, the removed tissues were solid neoplasm together with
cyst walls and tissue of the pituitary gland with non-neoplastic changes indicating that the neuroendocrine neoplasm was
present nearby RCCs and the pituitary gland in the sellar region.
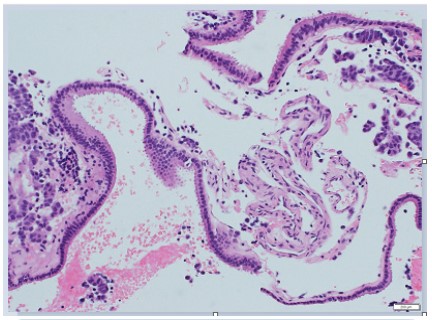
The cysts had lining by ciliated columnar or cuboidal epithelium
(Figure 2). Squamous cells or goblet cells were not recognized.
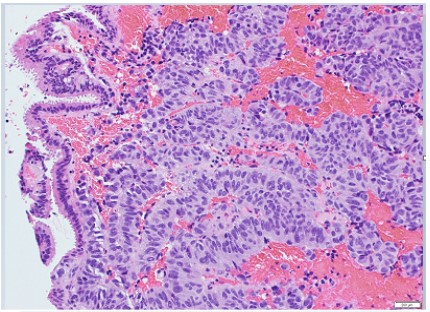
The cysts were diagnosed as RCCs. The tumor cells showed
the predominance of a trabecular pattern, often admixed with
tubuloacini or broad, irregular trabeculae with rosettes, and
only occasionally with solid nests (Figure 3). Cytologically, the
tumors possessed uniform round to oval nuclei with indistinct
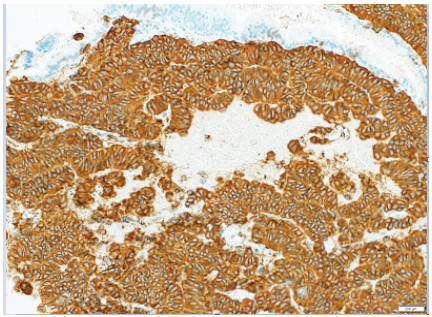
nucleori and coarsely granular chromatin pattern. Cellular mitosis was few and labeling index of KY67 was <2%. Immunohistochemically, the cells appeared strong activity of chromogranin
A and synaptophysin (Figure 4). The neoplasia was diagnosed
as a neuroendocrine neoplasm (carcinoid type) of low grade.
The lining epithelium of the cysts was frequently attached to
the margin of the neoplasm (Figure 3). After the operation, the
patient has been healthy without any symptoms of the sellar
lesion.
Discussion
In the present case of neuroendocrine neoplasm, histologi-
cal evaluation revealed that the tumor was a neuroendocrine
neoplasm of low grade with carcinoid-like pattern. Furthermore, no other primary or metastatic sites were detectable
suggesting that the neoplasm was primary. Up to date, only a
few cases of intracranial carcinoids have been reported [2-5].
Interestingly, these carcinoids were mainly dural-based tumors.
Furthermore, reported evidences suggest that carcinoid tumors
may have an affinity to dura mater on metastasis of other organs into the brain [13,14]. Thus, it is presumed that some of
the embryonic cells developing into the neuroendocrine tumors
may locate in the stromal tissues surrounding cerebrum including those of skull base. Furthermore, it is also suggested that
the neuroendocrine neoplasm like this case starts to grow from
the stromal cells in the sellar area.
Another possibility regarding the origin of the present neuroendocrine neoplasm would be certain potential cells migrating in the cyst wall of RCCs. According to Batt et al [15] with 30
cases RCCs, 56.6% of them had pituitary acini in their walls, and
5.6% of them constituted all the non-neoplastic cysts of central
nervous system including neuroepitheial element. Accordingly,
RCCs may have a role for development of neuroendocrine neoplasm as well as pituitary adenoma. Present case of collision of
carcinoid and RCCs could be a key issue for understanding of
clinicopathology of neuroendocrine neoplasm of the brain and
significance for the relationship between non-neoplastic cystic
lesions and neuroendocrine neoplasm in the central nervous
system.
References
- Maggard MA, O’Connel, JB, Ko CY, Updated population-based
review of carcinoid tumors. Ann Surg. 2004; 240: 117-122.
- Porter DG, Chakrabarty A, McEvoy A, et al, Intracranial carcinoid
without evidence of extracranial disease. Neuropathol Appl Neurobiol. 2000; 26: 298-300.
- Deshaies EM, Adamo MA, Qian J, DiRisio DA. A carcinoid tumor
mimicking an isolated intracranial meningioma. case report. J
Neurosurg. 2004; 101: 858-860.
- Ibrahim M, Yousef M, Bohnen N, Eisbruch A, Parmar H, Primary
carcinoid tumor of the skull base: case report and review of the
literature. J Neuroimaging. 2010; 20: 390-392.
- Hood B, Bray E, Bregy A, Norenberg M, Weed D, et al. Primary
carcinoid tumorof the cavernous sinus. World Neurosurgery.
2014; 81: 202-213.
- Voeker JL, Campbell RL, Muller J. Clinical radiographic, and
pathological features of symptomatic Rathke’s cleft cyst. J Neurosurg. 1977; 74: 535-544.
- Leech RW, Olafson RA. Epithelial cysts of neuraxis: presentation
of three cases and review of the origins and classification. Arch
Pathol Lab Med. 1977; 101: 196-202.
- Koutourousiou M, Kontogeorgos G, Wesseling P, Grotenhuis AJ,
Seretis A. Collision sellar lesions: experience with eight cases
and review of the literature. Pituitary. 2010; 13: 8-17.
- Lichtenberger FP, Glerean M, Paissan A, Ajler P. Collision sellar
lesions: pituitary adenoma and Rathke cleft cyst. Medicina (B Aires). 2021; 81: 1069-1072.
- Jagtap, VS, Lila AR, Sarathi V, et al, Coexistent pituitary adenoma
with Rathke’s cleft cyst: a case series. J Assoc Physicians India.
2018; 66: 42-46.
- Kepes JJ. Transitional cell tumour of the pituitary gland developing from a Rathke’s cleft cyst. Cancer. 1978; 41: 337-343.
- Ikeda H, Yoshimoto T, Katakura R. A case of Rathke’s cleft cyst
within a pituitary adenoma presenting with acromegaly-do
“transitional cell tumours of the pituitary gland” really exist?
Acta Neuropathol (Berl).1992; 83: 211-215.
- Kovachev D, Ruseva V. Carcinoid of the stomach with metastases
into the dura mater. Vutr Boles. 1976; 15: 98-103.
- Huang PS. Malignant carcinoid tumor metastatic to the dura mater simulating a meningima. Neurosurgery. 1991; 29: 449-452.
- Bhatt AS, Mhatre R, Nadeesh BN, Mahadevan A, Yasha TC, et
al. Nonneoplastic cystic lesion of the central nervous system-histomorphological spectrum: a study of 538 cases. J Neurosci
Rural Prac. 2019; 10: 494-501.