Introduction
It was reported that 27% of lung cancer patients were diagnosed with advanced stage by the time of detection with tumor
enlargement and necrosis, mediastinal lymphadenopathy, pleural, vascular or bronchial invasion, which challenges surgeons
and compromises surgical oncological outcome [1,2]. Video-Assisted Thoracic Surgery (VATS) in these cases is often very difficult and is occasionally converted to open surgery to ensure
R0 resection and adequate lymph node dissection.
Robot-Assisted Thoracic Surgery (RATS) has witnessed tremendous growth in the last two decades. For the treatment of
non-small cell lung cancer, compared with VATS, RATS lobectomy has been reported to decrease the rate of conversion to
open surgery, postoperative complications, and length of hospital stay [3-5]. RATS lobectomy benefits surgeons with flexibility
of robotic arms and intraoperative 3D imaging for better vascular and lymph node dissection, and therefore, has been shown
to be highly effective in advanced patients. Conversion rate to
open surgery range from 8.6% to 17.3%. postoperative complications are encountered in 27.6 - 44.2% of the cases, and the
30-day mortality rate is approximately 1.9% [6-8].
In Vietnam, RATS has only been recently developed. Currently, there are 5 operating Da Vinci Xi robotic surgery systems
nationwide. In thoracic surgery, Binh Dan Hospital was the first
to perform RATS lobectomy in July 2017, but the number of operations is still limited. At Cho Ray Hospital, since our first case
in July 2018, RATS lobectomy was developed rapidly with an
increasing number of patients. During the implementation of
RATS, we changed the position of the robotic arms approaching in a triangular shape to suit the local conditions with the
same approach criteria as VATS and reduced 01 robot arm.
From 7/2018 to 7/2022, we performed RATS lobectomy for 79
patients with stage I-IIIA disease. We conducted this study to
evaluate the efficacy of RATS lobectomy in patients with locally
advanced non-small cell lung cancer.
Method
Study design
From July 2018 to June 2022, RATS lobectomy was performed in 79 patients with non-small cell lung cancer at Cho
Ray Hospital, Vietnam. We included the patients diagnosed
with clinical stage I, II and IIIA (8th edition of TNM classification of the Internatonal Association of the study of Lung cancer)
via contrast chest CT scan, brain MRI and PET scan. These patients were candidates for radical surgical resection (ASA 1–3).
Exclusion criteria were severe heart disease, renal impairment,
any other serious comorbidities according to the investigator,
recent oncologic history (another malignant tumor within the
last 2 years), and previous chest surgery. In stage cIIIA, we chose
T3N1 and T4N0, and excluded T4 which invaded diaphragm,
heart and main bronchus.
We divided 79 patient in two groups:
- Group 1: 50 patients who had tumor <5 cm of diameter.
- Group 2: 29 patients who had tumor 5 cm of diameter (cT3
and cT4).
Preoperative staging included contrast-enhanced total body CT and FDG-PET. The standard functional evaluation included
ECG, cardiologic evaluation, pulmonary function tests, and preanesthesia evaluation.
In case of suspected mediastinal nodes, EBUS or mediastinoscopy was performed before resection. A preoperative diagnosis was obtained by CT-driven needle biopsy or endobronchial
biopsy. In the absence of a preoperative diagnosis, intraoperative lung cancer was confirmed with frozen section.
Operative approaches
All procedures were performed under general anesthesia
with a double-lumen endotracheal tube to deflate the diseased
lung, with patients in the lateraldecubitus position. DaVinci Robotic System Xi was used with a 30◦ camera and standard endoscopic staplers. Our RATS technique was modified from the
protocol posted by American Chest Surgery Association to fit
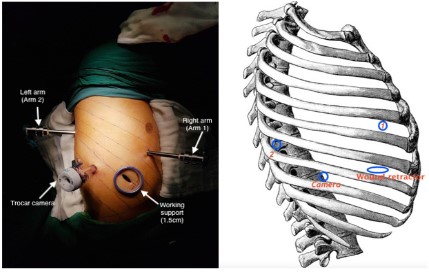
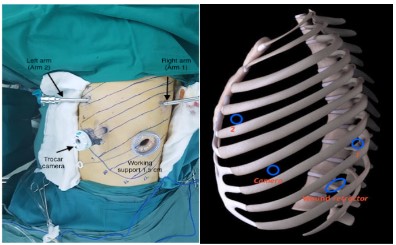
the circumstances in Vietnam. Robotic arms were set up as followed [4]:
- In case of right lung cancer: Camera trocar: 8th intercostal
space on the back 1 cm from posterior axillary line. Arm 1: 5th
intercostal space at the midpoint between anterior axillary line
and midclavicular line. Arm 2: 7th intercostal space on the back
3 cm from posterior axillary line. Assistant trocar (1.5 cm): 7th
intercostal space at anterior axillary line.
- In case of left lung cancer: Camera trocar: 7th intercostal
space at the midpoint between anterior axillary and midaxillary
line. Arm 1: 8th intercostal space on the back 3 cm from posterior axillary line. Arm 2: 4th intercostal space at the midpoint
between anterior axillary line and midclavicular line. Assistant
trocar: (1.5 cm): 9th intercostal space at anterior axillary line.
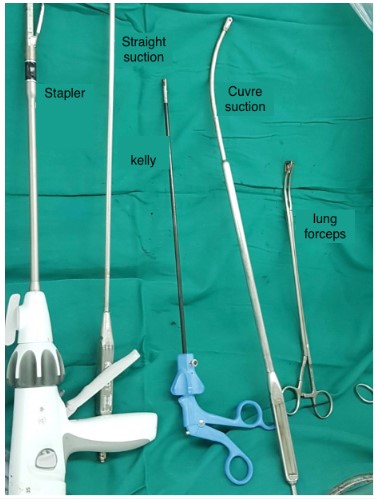
In all cases, we used only the cadiere forcep and harmonic
scalpel robotic arms which were further supported with thoracoscopic instruments through assistant trocar: suction, Kelly
forcep, stapler, etc. No CO2 insufflation was needed. After lobectomy, N1 lymph nodes were routinely dissected. For N2, we
performed lymphadenectomy to lymph nodes >1 cm on CT scan
or on intraoperative screen.
Early outcome was investigated by: operative time; rate of
intra-operative bleeding defined as blood loss >500 ml due to
vessel damage, the rate of conversion to open procedure; the
number of lymph nodes collected; the rate of post-operative
complications; and mortality rate.
Statistical analysis
The recorded data was collected and entered in a spread-sheet computer program (Microsoft Excel 2010), and then exported to data editor page of IBM SPSS version 22.0 (SPSS Inc.,
Chicago, Illinois, USA). Descriptive statistics and frequency distribution were calculated. Chi-square test was used for bivariate
associations. For all tests, confidence interval and p-value were
set at 95% and ≤ 0.05 respectively.
Results
From 7/2018 to 7/2022, we performed RATS lobectomy on
79 non-small cell lung cancer patients and divided them into 2
groups:
- Group 1: 50 patents withtumor size < 5 cm.
- Group 2: 29 patients with tumor size 5 cm.
Table 1: Characteristic of patients.
|
Group 1 (n=50) N(%) |
Group 2 (n=29) N(%) |
p value |
| Gender |
|
|
|
|
Male |
33 (66.0) |
21 (72.4) |
0.55* |
|
Female |
17 (34.0) |
8 (27.6) |
| Age (years) |
61.2 ± 8.4 |
61.1 ± 9.6 |
0.92** |
| Tumor size (cm) |
2.7 ± 0.9 |
6.1 ± 1.2 |
0.001** |
| Lobular lesion distribution |
|
|
|
|
LUL |
16 (32.0) |
12 (41.4) |
0.46* |
|
LLL |
5(10.0) |
4(13.8) |
|
RUL |
16(32.0) |
5(17.2) |
|
RML |
2(4.0) |
0(0) |
|
RLL |
11(22.0) |
8(27.6) |
| Location of tumor |
|
|
|
|
Peripheral |
44(88.0) |
25(86.2) |
0.81 |
|
Central |
6(12.0) |
4(13.8) |
| FEV1/FVC (%) |
77.2 ± 11.4 |
73.7 ± 9.2 |
0.19** |
| TNM staging (cTNM/pTNM) |
|
|
|
|
Stage I |
34 (68.0)/ 31 (62.0) |
0 (0) / 0 (0) |
0.001* |
|
Stage IIA |
2 (4.0) / 0 (0) |
0 (0) / (0) |
|
Stage IIB |
4 (8.0) / 10 (20.0) |
10 (34.5) / 17 (58.7) |
|
Stage IIIA |
10 (20.0) / 8 (16.0) |
19 (65.5) / 5 (17.2) |
|
Stage IIIB |
0 (0) / 1 (2.0) |
0 (0) / 7 (24.1) |
*: chi-quare test; **: t-test,
LUL: left upper lobe ; LLL: left lower lobe; RUL: right upper lobe; RML: right middle lobe; RLL: right lower lobe.
cTNM: clinical TNM; pTNM: pathologic TNM
Table 2: Results of operation.
|
Group 1 (n=50) N(%) |
Group 2 (n=29) N(%) |
p value |
| Operative time |
255.5 ± 68.4 |
273.7 ± 88.5 |
0.31** |
| N2 lymphadenectomy |
36 (72.0) |
23 (79.3) |
0.33* |
| Number of N2lymph nodes collected |
|
|
|
|
Station 1 |
22 (61.1) |
9 (39.1) |
0.11* |
|
Station 2 |
10 (27.8) |
7 (30.4) |
|
Station 3 |
3 (8.3) |
7 (30.4) |
|
Station 4 |
1 (2.8) |
0 (0) |
| Intra-operative bleeding |
1 (2.0) |
1 (3.4) |
0.6* |
| Conversion to open surgery |
2 (4.0) |
5 (17.2) |
0.046* |
| Post-operative complications |
|
|
|
|
Pneumonia |
1 (2.0) |
0 (0) |
0.07* |
|
Stroke |
1 (2.0) |
0 (0) |
|
Prolonged air leak (>7 days) |
2 (4.0) |
6 (20.7) |
|
Emphysema |
0 (0) |
1 (3.4) |
|
Bronchial fistula |
1 (2.0) |
0 (0) |
*: chi-quare test; **: t-test
Table 3: Results of operation.
|
|
Group 1 (n=50) N(%) |
Group 2 (n=29) N(%) |
p value |
| Pathological results |
|
|
|
|
Adenocarcinoma |
46 (92.0) |
25 (86.2) |
0.411* |
|
Squamous carcinoma |
4 (8.0) |
4 (13.8) |
| Metastatic lymph node level N1 |
12 (24.0) |
5 (17.2) |
0.481* |
| Metastatic lymph node level N2 |
9 (18.0) |
7 (23.1) |
0.513* |
| Time of follow up (month) |
26.2 ± 10.9 |
22.1 ± 9.4 |
0.323** |
| Recurrent lymph node |
14 (31.8) |
6 (27.3) |
0.705* |
| Distant metastasis |
17 (39.5) |
10 (45.5) |
0.674* |
*: chi-quare test; **: T-test
Table 4:
| Time of suvival |
Group 1 (n=50) N(%) |
Group 2 (n=29) N(%) |
p value |
| 1 year |
91.3 |
80.4 |
0.272* |
| 2 year |
88.0 |
62.2 |
*: log rank (Mantel-Cox)
There were no statistically significant differences in gender,
age, tumor location and preoperative respiratory function between 2 groups. In group 2, the clinical stage was mainly IIIA,
accounting for 65.5%. On CT scan images, group 2 had a significantly higher percentage of enlarged N1 and N2 lymph nodes
than group 1.
The mean operative time of the tumor 5 cm group was longer than that of the other group (273.7 minutes vs. 255.5 minutes), but the difference was not statistically significant. The
rate of lymph node dissection implemented in the 2 groups was
similar (group 1: 72% vs group 2 79.3%, p=0.33). In group 2, the
number of N2 lymphadenectomy performed at 2 or more stations accounted for 60.8%. The rate of intraoperative bleeding
was similar in the 2 groups. The rate of conversion to surgery in
group 2 was significantly higher than in group 1 (17.2% vs 4.0%)
(p = 0.046). In group 1, there were 2 cases of conversion to open
surgery, one of which was due to arterial damage during dissection. In group 2, all the 5 cases converted to elective open surgery was due to lack of space for manipulation or invasion
to the bronchi/blood vessels. The most common postoperative
complication was pneumothorax lasting >7 days, group 2 had a
complication rate of prolonged pneumothorax of 20.7%. There
was no statistically significant difference in postoperative complications in the 2 groups.
Most pathology findings in the two groups were adenocarcinoma. The rate of lymph node metastasis to N1 and N2 in
the 2 groups was insignificantly different. During postoperative
follow-up, we found that the rate of lymph node recurrence and
distant metastasis in the 2 groups had no statistically significant
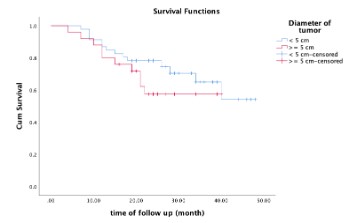
difference. In group 1, the survival rates were 91.3% and 80.4%
respectively after 1 and 2 years. In group 2, survival rate were
88% and 62.2% respectively. There was no significant difference
in the survival rate between the 2 groups (p=0.272).
Discussion
First, we would like to discuss about the modified triangualar
port placement in RATS lobectomy. Currently, there are 2 approaches in RATS: total and partial approach with robotic arms.
Parini et al also reviewed that there were many approaches and
positions of robotic arms to consider depending on the actual
conditions at the centers, the generation of robots used, and
surgeons’ habits and experience [9]. To the best of our knowledge, many authors changed the trocar placement of the robotic arm with different approaches, as mentioned in a study by
Veronesi G [10]. At our center, we choose partial-approach RATS
with one 1.5 cm assistant trocar for conventional thoracoscopic
instrument during surgery. With this modification, we saved 01
robotic arm, helping to reduce the cost of RATS (about 12-14
million VND per case). Surgeons are familarized with switching
from VATS to RATS and take advantage of the flexible robotic
arms in dissection and lobectomy.
There was no significant difference between the 2 groups in
terms of operative time. The mean operative time in the group
with tumors ≥5 cm was 273.7 minutes. Kneuertz P.J. recorded a
mean operative time of 283.6 minutes with RATS lobectomy in
296 patients [12]. Nelson B.D. et al reported their mean operative time on 106 patients was 226 minutes, significantly longer
than conventional thoracoscopy with 173 minutes (p<0.001)
[13]. The issue of prolonged operative time with RATS has also
been reported in many other multicenter studies.
A study by Mao J. et al in 2019 showed that RATS significantly
took longer than conventional laparoscopic surgery (p<0.001).
However, reports in the last 5 years showed no statistically significant difference between the 2 surgical groups in terms of
surgery time [14]. A meta-analysis by Ma J reviewing 13 reports
from 2015 to 2020 comparing operative time between RATS
and conventional laparoscopic surgery implied that there was
no statistically significant difference (p=0.92) [15].
In our study, the rate of conversion to open surgery in the
group of tumors >5 cm was 17.2%, all were due to large tumor
and vascular invasion, which minimized manipulation space and
made laparoscopic dissection difficult and therefore, compromised oncological outcome of the surgery. In the group of tumors < 5 cm, the rate of conversion to open surgery was 2/50
cases, one of which were due to pulmonary artery injury during dissection. The rate of conversion to surgery in group 2 was
higher than in group 1 (17.2% vs 4%) with statistical significance
with p = 0.046. Author Yang H-X et al (2016) reported the rate
of conversion to open surgery was 9.2% (16 cases) in RATS for
172 patients, with 3 cases due to bleeding (1.7%), 5 cases due
to adhesions in pleural space, 3 cases of inadequate ventilation
with single lun, 2 cases due to incompetent assistant, 1 case with limited intra-thoracic view, 01 case with anesthesia machine error and 01 case of bulky hilar lymph nodes [11]. Veronesi showed a conversion rate to open surgery of 15.2% of 223
patients with stage pIIIA non-small cell lung cancer [7]. Huang et
al. reported a conversion rate in 58 patients with stage cIIIA of
8.6% [8]. Except for the conversion to open surgery due to vascular injury, plan for anticipated conversion due to large tumor
or lymph node invasion should depend on the surgeons' level of
experience. Planned open surgery will help reduce blood loss
and ensure safety for the patients.
In our study, during postoperative follow-up, we found that
the rate of lymph node recurrence and distant metastasis in
the 2 groups had no statistically significant difference. The survival rates for group 1 were 91.3% and 80.4%, respectively after 1 and 2 years. In group 2, survival rates were respectively
88% and 62.2%. There was no significant difference in survival
between the 2 groups (p=0.272). Author Cerfolio conducted
a study on 1339 patients undergoing RATS lobectomy and recorded the 5-year survival rates by stage as follows: IA 83%, IB
77%, IIA 68%, IIB 70 %, IIIA 62% (N2 metastasis 73%), IIIB 31%
(without N3 metastasis). The author also emphasizes that the
excellent survival rate of RATS is due to its ability to radically
remove lymph nodes, improving the pathologic staging and
thereby, more appropriate and early decision for adjuvant chemotherapy at the correct stage [16]. A report on robotic non-small cell lung cancer surgery on 249 patients by Toosi et al
showed a mean follow-up time of 18 months. The lung cancer
stage survival rates assessed after surgery at 1 year and 3 years
were: Stage-I, 92% (87–97%) and 75% (63–87%); Stage-II, 83%
(70–96%) and 73% (49–97%); Stage-III, 75% (63–87%) and 44%
(26–62%); and Stage-IV, 67% (37–97%) and 0% [17] . The survival rate in our study is similar to that of other authors in the
world.
Conclusion
RATS is effective in lobectomy for non-small cell lung cancer
5 cm in size. Tumor size 5 cm did not increase the surgical time,
the rate of postoperative complications, or change the postoperative recurrence rate. The rate of conversion to open surgery
increased when the tumor is 5cm and the decision of conversion was within the plan.
References
- Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National
Cancer Database survey. J Thorac Oncol. 2010; 5: 29-33.
- Bryan DS, Donington JS. The Role of Surgery in Management of
Locally Advanced Non-Small Cell Lung Cancer. Curr Treat Options Oncol. 2019; 20: 27
- Wei S, Chen M, Chen N, Liu L. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis.
World J Surg Oncol. 2017; 15: 98
- Emmert A, Straube C, Buentzel J, Roever C. Robotic versus thoracoscopic lung resection: A systematic review and meta-analysis.
Medicine (Baltimore). 2017; 96: e763.
- Oh DS, Reddy RM, Gorrepati ML, Mehendale S, Reed MF. “Robotic- assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data”. Ann
Thorac Surg. 2017; 104: 1733-1740.
- Glover J, Velez-Cubian FO, Toosi K, Ng E, Moodie CC, et al. Peri-operative outcomes and lymph node assessment after induction therapy in patients with clinical N1 or N2 non-small cell lung
cancer. J Thorac Dis. 2016; 8: 2165-2174.
- Veronesi G, Park B, Cerfolio R, Dylewski M, Toker A, et al. Robotic
resection of Stage III lung cancer: an international retrospective
study. Eur J Cardiothorac Surg. 2018; 54: 912-919.
- Huang J, Li C, Li H, Lv F, Jiang L, et al. Robot-assisted thoracoscopic surgery versus thoracotomy for c-N2 stage NSCLC: short-term
outcomes of a randomized trial. Transl Lung Cancer Res. 2019; 8: 951-958.
- Parini S, Massera F, Papalia E, Baietto G, Bora G, et al. Placement Strategies for Robotic Pulmonary Lobectomy: A Narrative
Review. J Clin Med. 2022; 11: 2612.
- Veronesi G, Abbas AE, Murianna P, Lembo R, Bottoni E, et al.
Perioperative outcome of robotic approach versus manual videothoracoscopic major resection in patients affected by early
lung cancer: resultss od a randomized multicentric study (ROMAN study). Front Oncol. 2021; 11: 726408.
- Yang HX, Woo KM, Sima CS, Bains MS, Adusumilli PS, et al. Long
term survival based on the surgical approach to lobectomy for
clinical stage I nonsmall cell lung caner: comparison of robotic,
video-assited thoracic surgery and thoracotomy lobectomy. Ann
Surg. 2016.
- Kneuertz PJ, Singer E, D’Souza H, Abdel-Rasoul M, Moffatt-Bruce
SD, et al. “Hospital cost and clinical effective- ness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy:
A propensity score-weighted comparison. J Thorac Cardiovasc
Surg. 2019; 157: 2018-2026.e2.
- Nelson DB, Mehran RJ, Mitchell KG, Rajaram R, Correa AM, et
al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer:
A Comprehensive Institutional Experience. Ann Thorac Surg.
2019; 108: 370-376.
- Mao J, Tang Z, Mi Y, Xu H, Li K, et al. Robotic and video-assisted
lobectomy/segmentectomy for non-small cell lung cancer have
similar perioperative outcomes: a systematic review and meta-analysis. Translational Cancer Research. 2021; 10: 3883-3893.
- Ma J, Li X, Zhao S, Wang J, Zhang W, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non – small cell lung
cancer: a meta-analysis. BMC cancer. 2021; 21: 498.
- Cefolio RJ, Ghanim AF, Dylewsko M, Veronesi G, Spaggiari L, et
al. The long-term survival of robotic lobectomy for non-small
cell lung cancer: a multi-institutional study. J Thorac Cardiovasc
Surg. 2018; 155: 778-786.
- Toosi K, Velez-Cubian FO, Glover J, Ng EP, Moodie CC, et al. Up-staging and survival after robotic-assissted thoracoscopic lobectomy for non-small cell lung cancer. Surgery. 2016; 160: 1211-1218.